
Pivot Signs Option Agreement to Acquire Licensing Rights to Patented TriVair™ Nasal and Pulmonary Drug Delivery System For Its “RTIC” Ready-To-Infuse-Cannabis Powder
PR Newswire
VANCOUVER, May 31, 2018
VANCOUVER, May 31, 2018 /PRNewswire/ – Pivot Pharmaceuticals Inc. (CSE: PVOT / OTCQB: PVOTF / FRA: NPAT) (“Pivot” or the “Company”) is pleased to announce that the Company has signed an Option Agreement with IP Med Inc., based in Oceanside, New York, to acquire an exclusive worldwide license for Trivair™ Nasal and Pulmonary Breath-Propelled Drug Delivery Systems™ for the delivery of Pivot’s Ready-To-Infuse-Cannabis (“RTIC”) cannabinoid products. The Option expires in 6-months during which time Pivot will determine compatibility with Pivot’s RTIC powder formulations. Upon successful completion of the evaluation Pivot will have the Option to enter into a Definitive Licensing Agreement with IP Med for the TriVair™ device.
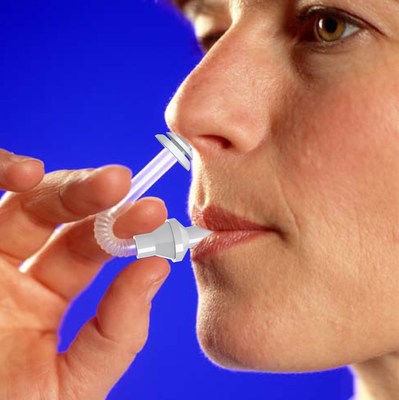
Winner of the Frost and Sullivan 2009 European Drug Delivery Product Differentiation Innovation of the Year Award, the intuitive and easy-to-use TriVair™ patented devices are discreet and disposable, deliver pre-loaded precise unit doses and eliminate the risk of microbial contamination. Clinical studies using the TriVair™ Delivery System have demonstrated that the powder compound is widely distributed in all areas of the nasal cavity (with no area of powder accumulation), is effective in retaining medication in the nasal cavity without pharyngeal penetration and showed a high degree of patient acceptability.
The TriVair™ Nasal device uses the body’s natural breath to propel a medication deep into the nasal cavity to targeted sites, bypassing the Blood Brain Barrier, providing the potential to better treat central nervous system diseases such as migraines, epilepsy, insomnia, and post-operative pain, the latter of which is often remedied by opioids. The TriVair™ Pulmonary device enables specific dosing to the pulmonary airways, thereby targeting the lungs. Such targeting can be highly relevant in the treatment of respiratory diseases and in the prevention and treatment of respiratory infections.
Pivot will combine its patented RTIC™ cannabis powder technology with the TriVair™ delivery system to provide a rapid onset effect for patients and consumers in markets where regulations permit. Pivot’s Product Formulation Team, led by Dr. Leonid Lurya in Israel, has initiated testing to evaluate, modify and test formulations that achieve the desired particle size for delivery by the TriVair™ device.
IP Med’s Vice-President, Mr. Ben Isaacs, comments that “I am excited to partner with the team at Pivot Pharmaceuticals. With their product development and commercialization experience, and impressive RTIC powdered cannabis technology I am confident that the TriVair™ device will provide a safe and effective way for the delivery of cannabinoids into patients.”
Dr. Joseph Borovsky, Pivot’s Executive Vice President, Product Development, stated “We are very pleased to add the TriVair™ device to Pivot’s product line and expand the range of delivery systems for cannabinoids. This pre-loaded and single-use device, will be a disruptive technology and provide solutions for patients with special medical needs.”
Pivot’s Chief Medical Officer, Dr. Wolfgang Renz commented “Pivot continues to grow its portfolio of patented technologies specifically for the formulation and delivery of cannabinoids. We are very excited about the unique opportunity this differentiated product presents for patients seeking an alternative method to traditional cannabis practices. Upon the completion of our compatibility testing, Pivot will have the world’s first nasal and pulmonary powdered cannabis device for quick onset and use in the medical field. This combination product may be well suited for managing such indications as sleep disorder, migraine, late-stage cancer pain, and address opioid withdrawal.”



About IP Med Inc.
IP Med partners with (physician) inventors, academic institutions and pharmaceutical companies to bring their concepts to market: We create, develop (design and engineer), patent, prototype, build proof of concept, commercialize and/or monetize unique and innovative technologies and compounds in efficient and cost effective ways. IP Med’s founders bring 35 + years of success and experience in strategic planning and marketing for the pharmaceutical and medical product industries; working with top-tier companies including Pfizer, Wyeth, Eli Lilly, Merck, AstraZeneca and GlaxoSmithKline just to name a few. IP Med maintains close relationships with a vast network of executives within major companies in the medical arena; including pharmaceutical, biotech and medical device companies. For more information please visit www.ipmedinc.com
About Pivot Pharmaceuticals Inc.
Pivot Pharmaceuticals Inc. is a biopharmaceutical company engaged in the development and commercialization of therapeutic pharmaceuticals and nutraceuticals using innovative drug delivery platform technologies. Pivot’s wholly-owned medical cannabis products division, Pivot Green Stream Health Solutions Inc. (“PGS” or “Pivot Green Stream”), conducts research, development and commercialization of cannabinoid-based nutraceuticals and pharmaceuticals. Pivot’s wholly-owned U.S. subsidiary, Pivot Naturals, LLC, based in Costa Mesa, California, will manufacture and supply finished powderized cannabis products such as food additives, capsules, bulk powder and stick packs to the California market. PGS has acquired worldwide rights to “RTIC” Ready-To-Infuse Cannabis oil-to-powder technology, BiPhasix™ Dermal Drug Delivery platform technology (topical), Solmic Solubilisation technology (oral) and Thrudermic Transdermal Nanotechnology (transdermal) for the delivery and commercialization of cannabinoid, cannabidiol (CBD), and tetrahydrocannabinol (THC)-based products. For more information please visit www.PivotPharma.com
Cautionary Statement
Except for historical information contained herein, the matters set forth above may be forward-looking statements that involve certain risks and uncertainties that could cause actual results to differ from those in the forward-looking statements. Words such as anticipate, believe, estimate, expect, intend, and similar expressions, as they relate to Pivot Pharmaceuticals Inc., Pivot Green Stream Health Solutions Inc., Pivot Naturals, LLC, IP Med Inc., or its management, identify forward-looking statements. Such forward-looking statements are based on the current beliefs of management, as well as assumptions made by and information currently available to management. Actual results could differ materially from those contemplated by the forward-looking statements as a result of certain factors, such as the failure to meet the conditions imposed by the CSE or other securities regulators, the level of business and consumer spending, the amount of sales of Pivot’s products, statements with respect to internal expectations, including with respect to the TriVair™ device, the competitive environment within the industry, the ability of Pivot to continue to expand its operations, the level of costs incurred in connection with Pivot’s expansion efforts, economic conditions in the industry, and the financial strength of Pivot’s customers and suppliers. Pivot does not undertake any obligation to update such forward-looking statements. Investors are also directed to consider all other risks and uncertainties.
![]() View original content with multimedia:http://www.prnewswire.com/news-releases/pivot-signs-option-agreement-to-acquire-licensing-rights-to-patented-trivair-nasal-and-pulmonary-drug-delivery-system-for-its-rtic-ready-to-infuse-cannabis-powder-300657112.html
View original content with multimedia:http://www.prnewswire.com/news-releases/pivot-signs-option-agreement-to-acquire-licensing-rights-to-patented-trivair-nasal-and-pulmonary-drug-delivery-system-for-its-rtic-ready-to-infuse-cannabis-powder-300657112.html
SOURCE Pivot Pharmaceuticals Inc.



Be the first to comment