
Vitacon Receives FDA 510(k) Clearance and Creates US Presence
PR Newswire
MINNEAPOLIS, Nov. 16, 2012
MINNEAPOLIS, Nov. 16, 2012 /PRNewswire/ — Vitacon AS, located in Trondheim, Norway, announces major milestones as it continues to expand its global reach. Vitacon has received FDA clearance on its VitaScan LT USB bladder scanner. Vitacon also announces signing a marketing and distribution partnership with SALUS, the Minneapolis-based life science advisory firm.
Non-invasive bladder scanning has become a valuable diagnostic tool. Measuring bladder volume helps avoid indwelling urethral catheters, a major cause of infection. CAUTI (catheter-associated urinary tract infection) treatment is no longer reimbursed if it happens while a patient is hospitalized, thus a major incentive exists to prevent it.
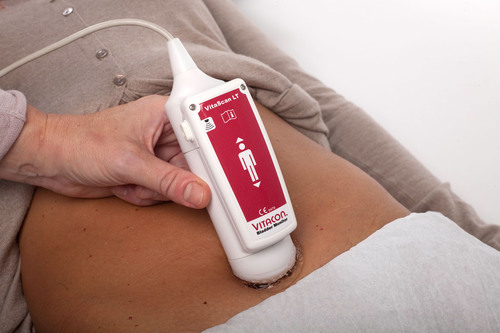
VitaScan LT enables real-time bladder volume measurement in hospitals, clinics, and home health. It connects via USB to laptop and tablets, providing nearly limitless storage and data transmission capabilities. VitaScan software utilizes intelligent targeting algorithms enabling personnel with limited training to obtain accurate measurements in seconds.
SALUS was selected by Vitacon to implement a US marketing and distribution strategy. “The selection of SALUS is essential to our growth,” says Kristin Waro, CEO of Vitacon. “We found their approach unique as they not only created a thorough strategy, but also have the expertise and distribution connections to execute the plan.”
SALUS will be responsible for pre-sales support, logistics, and customer service for a nationwide network of distributors. Spencer Lien, SALUS CEO, says, “Vitacon hit our market perfectly, responding to the need for speed, mobility, and cost-effectiveness. Our research indicates a void in the marketplace and we are confident the respective channels to healthcare providers will be equally impressed.”
Vitacon AS was founded in 1985 in Trondheim, Norway. Vitacon develops products to treat incontinence and diagnose bladder dysfunction. Vitacon products are well-known throughout Europe. The company has moved into diagnostic ultrasound and recently launched the VitaScan series of ultrasound scanners offering flexible and cost-effective solutions in bladder volume measurements.
About SALUS:
Salus Consilium, LLC was founded in 2008 by veteran medtech entrepreneur, Spencer Lien. The firm specializes in advising new medical technology ventures and established companies on growth strategies and technology management. Headquartered in Minnesota‘s “Medical Alley,” SALUS offers a variety of services including business development, regulatory and reimbursement strategies, commercialization plans, intellectual property management, and technology transfer.
Website: www.vitacon.com
Website: www.TeamSalus.com
SOURCE Salus Consilium, LLC



Be the first to comment