
MediValve, Ltd., Announces Clearance of a 510(k) Pre-Marketing Notification with the US Food and Drug Administration for the acWire™ Guidewire
PR Newswire
KIBBUTZ HAMA’APIL, Israel, January 13, 2014
KIBBUTZ HAMA’APIL, Israel, January 13, 2014 /PRNewswire/ —
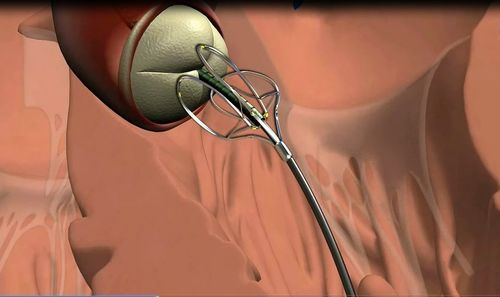
The acWire™ is intended for use in peripheral vascular and heart catheterization procedures to introduce and assist in positioning diagnostic and interventional devices. The acWire may also function as an alignment tool by providing a reference plane of anatomical structure of interest (i.e., the aortic valve).
MediValve, Ltd., a Trendlines portfolio company, today announced that it has received clearance of a Pre-Marketing Notification Application (510(k)) from the US Food and Drug Administration (FDA) for its acWire Guidewire[1],[2]. The acWire is intended for use in peripheral vascular and heart catheterization procedures to introduce and assist in positioning diagnostic and interventional devices. The acWire may also function as an alignment tool by providing a reference plane of anatomical structure of interest (i.e., the aortic valve). MediValve previously announced clearance of a CE pre-market application for the acWire.
Assaf Klein, Chief Executive Officer for MediValve, commented, “We are thrilled to have reached the milestone of FDA pre-market clearance for our 510(k) application. In today’s challenging regulatory environment it is extremely exciting for a young company to achieve both CE and FDA pre-market clearances in the same month. We anticipate significant clinical interest for this innovative technology. This is the second in a series of regulatory applications as we continue our development of several additional acWire products.”
Carlos E. Ruiz, M.D., Ph.D.[3], Professor and Chief of Structural and Congenital Heart Disease at North Shore-LIJ Hofstra University North Shore Health System and Lenox Hill Hospital of New York, said, “I am impressed with the acWire technology and the promise it holds for positioning diagnostic and interventional devices during heart catheterization procedures. I look forward to evaluating the acWire in our clinic.”
————————————————–
1. acWire has received CE Pre-market clearance November 08, 2013.
2. Patents pending.
3. Dr. Keren is a member of the MediValve Clinical Advisory Board.
About Carlos Ruiz, MD
Dr. Ruiz was born and raised in Barcelona, and completed his medical degree at the University of Barcelona. He trained at the Mayo Clinic with Dr. Valentin Fuster and the University of Southern California, where he did a Critical Care Fellowship with Dr. Harry Max Weil, and in 1991 was named the Director of the Pediatric and Adult Catheterization Labs at Loma Linda University Medical Center and Children’s Hospital. In 1999 he moved to Chicago where he was Chief of Pediatric Cardiology at the University of Illinois, Chicago until 2006 when he moved to Lenox Hill Heart and Vascular Institute of New York and New York University in New York City. He is currently Professor and Chief of Congenital & Structural Heart Disease for North Shore LIJ Health System. Dr. Ruiz is full professor of pediatrics and medicine. He has served on the editorial board of many prestigious cardiology journals, and has published over 250 manuscripts in peer reviewed journals in addition to many book chapters.
MediValve previously announced on January 06, 2014 CE pre-market clearance for the for the acWire™ Guidewire.
About the MediValve, Ltd., acWire™ Guidewire
The MediValve acWire Guidewire is a single-use, fully disposable medical device utilizing innovative shape-memory alloy technology intended to enable identification of cardiovascular structures utilizing existing imaging methods. Use of acWire employs methods and techniques currently utilized by interventional cardiologists to access the cardiovascular system. Once directed to a selected cardiovascular location, acWire is deformable under fluoroscopy to identify a desired specific anatomical landmark for subsequent therapeutic treatment by the clinician. AcWire is designed to facilitate the accurate placement and alignment of medical devices in the cardiovascular system during diagnostic and interventional procedures.
About Lenox Hill Hospital
Lenox Hill Hospital, a 652-bed, acute care hospital located on Manhattan’s Upper East Side, has earned a national reputation for outstanding patient care and innovative medical and surgical treatments. The hospital is particularly well known for excellence in internal medicine, cardiovascular disease, orthopaedics, sports medicine, otolaryngology/head and neck surgery, and maternal/child health. The Hospital is also a recognized leader in public health education and community outreach.
About MediValve Ltd.
MediValve, Ltd., was founded in 2010, it is a portfolio company of The Trendlines Group (http://www.trendlines.com). MediValve is developing the acWire Guidewire to facilitate the accurate placement of medical devices in the cardiovascular system during diagnostic and interventional procedures.
About Trendlines
The Trendlines Group (http://www.trendlines.com) creates and develops companies to improve the human condition, investing in medical device and agricultural technology companies. The Trendlines Group owns two Israel-based business incubators: Trendlines Medical, which invests medical device companies; and Trendlines Agtech, which focuses on agricultural technologies. The Trendlines portfolio includes 60 companies, with 15 that have already brought products to market.
For Further Information:
Bill Edelman, Chairman of the Board
MediValve, Ltd.
+1-781-436-0509
bill.edelman@medivalve.com
http://www.medivalve.com
SOURCE MediValve, Ltd.



Be the first to comment